“My back hurts a lot more than it did when I was younger. Well, I guess that’s the second law of thermodynamics at work.” Actually, it isn’t. Misconceptions of the second law of thermodynamics abound, even within the creationist community. Such misconceptions include the idea that the second law (1) is the tendency of any ordered system to go to disorder, (2) is the same as the curse associated with Adam’s sin, or at least began at the fall, (3) disallows order spontaneously arising from disorder, and (4) would obviously make Darwinian evolution impossible. None of these are true. But the second law is relevant to origins and can be powerful when used properly. To understand the second law, we need some background information in the field of thermodynamics.
Energy
First, we introduce the concept of energy. Energy is associated with motion. If something is moving, then it has energy. Energy of motion is called kinetic energy. Quantitatively, the kinetic energy of an object is half its mass multiplied by the square of its velocity.[1] So the faster something moves, the more kinetic energy it has. And an object of large mass will have more kinetic energy than a less massive object at a given speed.
Alternatively, if something has the ability to produce motion, even if it is not in motion, then it also has energy. This “stored” energy is called potential energy. A battery has potential energy because even if it is not in motion, it has the ability to produce motion when connected to an electrical motor. There are many different kinds of potential energy; chemical, electrical, gravitational, and so on.
Imagine dropping a bowling ball on concrete. Before you let go of the ball, it has potential energy. This is due to its position above the Earth’s surface and the force of gravity between the ball and the Earth. This is gravitational potential energy. When you let go of the ball its potential energy begins to convert to kinetic energy; the ball begins to move downward. Just before the ball strikes the concrete, it has a lot of kinetic energy, but its potential energy has been reduced because it is not as high above the Earth’s surface. In fact, the total energy of the ball throughout its journey has remained exactly the same.[2] This is a consequence of the first law of thermodynamics, which states that energy cannot be created nor destroyed, merely transformed from one type to another.[3]
But what happens to this energy when the ball strikes the concrete? The motion has stopped; the bowling ball is stationary. From the first law of thermodynamics, we know that the energy has not simply disappeared. Yet we no longer see motion. Did the energy convert back to some form of potential energy? In fact, the energy is still kinetic energy, but we cannot see it because it is on the atomic scale. The atoms and molecules of the bowling ball are vibrating and are therefore in motion. But their motions are randomized. In other words, some molecules are moving up while others are moving down, and some are moving to the left while others are moving to the right. So on the large scale these motions average out and the overall speed of the bowling ball is zero. We call this type of randomized energy on the atomic scale thermal energy.
So, thermal energy is a specific type of kinetic energy: that of the unseen randomized motion of the atoms or molecules. Just before the bowling ball hits the ground, all the molecules are moving in basically the same direction: down. But after it hits the ground, the molecular motion becomes randomized – they are all moving in different directions. Actually, the average speed of the molecules doesn’t change; they have the same speed after the ball hits the ground as they do before.[4] It’s just that their organization has changed from being uniformly down to being randomized.
This randomized motion of molecules is thermal energy which we recognize as temperature. Hotter objects have greater thermal energy than colder objects of equal mass because the molecules are vibrating faster in the hotter object. So, after the bowling ball strikes the ground, it will be (slightly) warmer than it was before. Its temperature has increased. Thermal energy is kinetic energy that you cannot see because it involves the randomized motions of atoms and molecules.
Thermodynamics is the study of thermal energy and how it flows. When two objects of different temperature are placed in thermal contact, the net flow of energy will always spontaneously flow from the hotter object to the cooler one. As it gains energy, the colder object tends to heat up while the warmer object cools off. Eventually, the two objects will come to the same temperature.
Consider an ice cube placed in hot coffee. The molecules in the ice cube are moving relatively slowly, whereas the molecules in the coffee are moving rapidly. As the rapid molecules strike the slower molecules, they impart some of their energy. The slower molecules are sped up while the faster molecules are slowed down. This process will continue until all the molecules have about the same kinetic energy – a condition we call thermal equilibrium. Hence, eventually you end up with lukewarm coffee.
The flow of thermal energy is called heat. We say that the coffee has heated the ice cube causing the latter to melt and cool the coffee. The flow of heat is naturally from the hotter material to the cooler material. Eventually, the cup of coffee has the same temperature everywhere.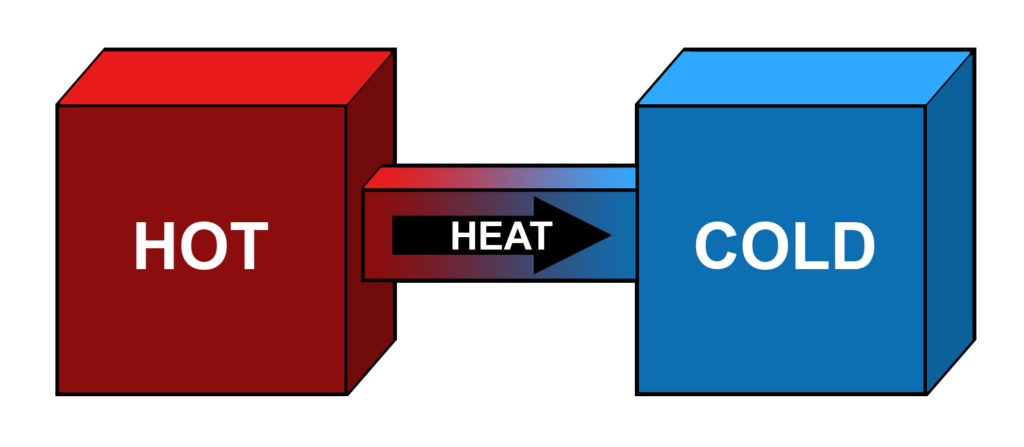 The Second Law
The Second Law
This is the second law of thermodynamics. Namely, the net flow of thermal energy in an isolated system will always be from the warmer to the cooler and never the reverse; entropy will increase until equilibrium is achieved. You will never start with lukewarm coffee, and then see an ice cube spontaneously form in the middle while the coffee becomes hot. The net flow of thermal energy is always from the hotter object to the colder one in any isolated system. Objects in thermal contact will naturally, eventually, come to the same temperature.
Physicists use the term entropy to describe the randomness of atomic or molecular motions. You can think of entropy as a measure of the messed-up-ness of atomic motions. The concept is sort of backwards from our intuition because atoms whose motion is well-organized and uniform have less entropy than atoms whose motion is disorganized. Temperature differences represent a low-entropy situation because there is organization when the faster molecules are separated from the slower ones. Energy will tend to flow from a hotter object to a cooler one, and this flow of energy can be used to do useful work, to make something move. But eventually, the two objects reach equilibrium: they come to the same temperature.
Once the system has reached equilibrium, the entropy has reached maximum and the energy can no longer be used to do any useful work. So, low entropy energy can be used to do work, which increases the entropy of the energy until it is useless. Energy is constantly going from a useful form to a less useful (and ultimately unusable) form. For this reason, the second law of thermodynamics is also called the law of entropy.
There are three primary ways that energy will flow from hotter sources to colder sinks: conduction, convection, and radiation. Conduction involves the direct collision of molecules. So the example of the ice cube in hot coffee involves energy exchange by conduction. Convection involves bulk movement of the material usually due to density differences. Hot air is lighter than cold air of equal composition and pressure, and therefore tends to move up, transporting its energy. This process is generally much faster than conduction.
However, even if two objects are not touching, they can still exchange energy. Imagine an ice cube separated from hot coffee by a vacuum. They would eventually come to the same temperature by radiation. Some of the energy of the hot coffee would convert to photons (particles of light) in the infrared portion of the spectrum which we cannot see. They travel away cooling the coffee. Some of these photons would travel to the ice cube and deliver their energy, heating the ice.[5] The thermal energy from the sun is delivered to Earth by radiation.
The Usefulness of the Second Law
Many man-made machines use the second law of thermodynamics to operate. Since thermal energy will spontaneously flow from a hotter object to a colder one, we can use that heat to perform useful work. For example, a thermocouple can use temperature differences to create electricity. Mechanical engines work by dumping heat from a hotter source to a colder environment, and use that to produce motion.
Perhaps the most ironic use of heat spontaneously going from hot to cold is to force (a smaller amount of) heat to go the other way. An air conditioner cools your home by pumping thermal energy from the cooler interior to the hotter exterior – the opposite way that the energy would spontaneously flow. Does this violate the second law of thermodynamics? The second law states that the net flow of energy in an isolated system will go from the hotter object to the cooler, until the two objects reach equilibrium. An air conditioner is not an isolated system, so there is no violation.
An isolated system is one in which no energy is allowed in or out. Your home is not isolated because you have electricity flowing into it. This electricity has been produced by some process in which heat has gone from hot to cold – the normal way. This allows you to power a device that will force some thermal energy to go the opposite of its normal direction. But it will be less than the thermal energy that has gone from hot to cold. An analogy might be helpful.
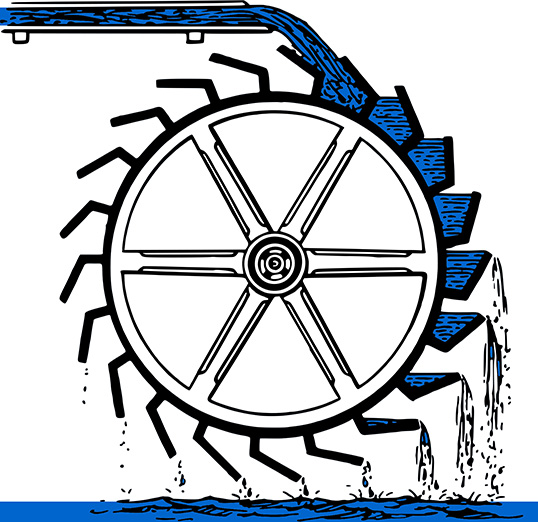
Imagine a waterwheel. This device uses the natural tendency of water to flow downhill to do useful work. As the stream flows over the wheel, some of the water’s gravitational potential energy is used to turn the wheel and produce kinetic energy. The kinetic energy of the wheel can be used to do useful work. One thing you could use the wheel to do is to lift water somewhere else. You could connect the wheel via a shaft to a smaller wheel that lifts water from a lower level and deposits it at a higher level. In other words, you can mechanically force some water uphill by allowing a much larger amount of water to flow downhill. But if you tried to lift more water uphill than is flowing downhill in the stream, it won’t work. The wheel would stop turning. The overall flow of water must be downhill.
Likewise, you can locally decrease entropy by pumping heat from the cooler interior of your home to the hotter exterior. But this only works because the power plant producing the electricity has allowed a much greater amount of heat to flow from hot to cold. By locally decreasing the entropy of your home, you have increased the entropy of the rest of the universe by a greater amount. The second law of thermodynamics does allow local decreases of entropy so long as the entropy of the entire (isolated) system increases.
The Second Law is a Thermodynamic Law
We already have enough information to refute some of the myths surrounding the second law of thermodynamics. Being a law of thermodynamics, it deals with heat. It has nothing to do with non-heat related phenomena, such as aging, or order in a generic sense. Shuffling a brand-new deck of cards will decrease their orderliness; but the order of cards is unrelated to their temperature and therefore is not related to the second law of thermodynamics.
The second law describes the net flow of thermal energy in an isolated system. It does not explain why your home tends to go from an ordered state to a disordered one. It does not forbid a disordered state from becoming ordered. If you mix oil and water and let the mixture sit, the oil will spontaneously separate from the water, going from a mixed, disordered state to an ordered one. This has nothing to do with the second law of thermodynamics, because it does not involve heat.
Some people have made the argument that particles-to-people evolution violates the second law of thermodynamics. They say that people are more organized and complex than particles and the simple never spontaneously becomes complex. The problem is that this is not a thermodynamic issue; it does not involve the flow of heat and is therefore irrelevant to the second law. A human might have the same temperature as the molecules from which he or she supposedly evolved. And so there is no obvious violation of the second law of thermodynamics.
To be clear, there are lots of great reasons to think that neo-Darwinian evolution is impossible. But unless a person can show that such evolution violates the condition that the net flow of thermal energy must be from hot to cold in an isolate system, then the second law is not violated. Perhaps an argument can be made showing the impossibility of the chance formation of DNA or similar life-essential molecules in which the second law is used as part of the argument. But we cannot simply dismiss evolution as violating the second law without showing that it really does. And the fact that life supposedly evolved in a system that is not even remotely isolated might make such an argument difficult to formulate.
It’s a Good Law
The second law of thermodynamics is essential for life. The chemical reactions in our body produce heat. But since our body is warmer than the surrounding air, the thermal energy will naturally flow from our body to the surrounding air (both by conduction and by radiation), heating the air and cooling our body. Without the second law of thermodynamics we would boil to death in minutes.
The second law is also responsible for maintaining the Earth’s temperature, and keeping it just right for life. The Earth is warmer than the vacuum of space, and therefore is constantly radiating away its energy into space. But the sun is much hotter than the Earth, and therefore the net flow of energy is from the sun to the Earth. So the reason we receive light and heat from the sun is the second law of thermodynamics. Apart from this law, the light and heat would be just as likely to go the other way – from the Earth to the Sun, in which case the Earth would freeze.
Not the Curse
So we see that the second law of thermodynamics is a very good thing and essential for life. It is therefore not the result of the curse since life was possible before the curse. Furthermore, we have textual evidence from Genesis that the second law of thermodynamics was in operation before the curse. Recall that light energy goes from the hotter sun to the colder Earth as a result of the second law. Was this the case before Adam sinned? Yes, Genesis 1:17 states that the purpose of the luminaries was to give light upon the Earth, and that it was so, which means the net flow of the light energy was from the sun to the Earth. This was on the fourth day of creation – before the curse. Clearly, the second law was already in operation.
Why then did some creationists of the past think that the second law started at the curse? There are perhaps three reasons for this. First, some people have the impression that entropy is somehow bad. After all, entropy is a measure of the mixed-up-ness of the motions of the atoms. And high-entropy energy is useless to us because it cannot be used to do work. But how is that a bad thing? It would be bad if the Earth had no way of replenishing its high-entropy energy with low-entropy energy. But in fact, God has ensured that the Earth will have usable energy for the foreseeable future. The second law of thermodynamics causes the Earth to constantly dump its useless high-entropy energy to space, and to receive a fresh supply of low-entropy energy from the sun. All life is apparently designed to use this process. So there is no problem there.
A second reason might be that the conversion from low-entropy usable energy to high-entropy useless energy might be seen as a type of decay. And doesn’t the Bible teach that decay was introduced at the time of Adam’s sin? The NIV translation of Romans 8:21 makes clear that the present world suffers a “bondage to decay” as a result of the curse. However, this passage is not referring to thermal decay nor decay in a generic sense, but rather to spiritual decay: the degradation of our character due to sin – corruption. Most other English translations render the phrase “bondage of corruption” which perhaps better conveys the idea. Namely, it is our sin-nature, our tendency to rebel against God, for which we eagerly await deliverance.
But other types of decay are good and are not the result of sin. Adam and Eve were to eat food even before sin (Genesis 1:29). And the digestion of food is a type of decay – one for which we should be very grateful. These are good processes that God has instilled in nature by which waste decays and is recycled back into the environment.
There is a third reason that deserves careful consideration. The second law implies that energy is constantly going from a usable to useless form. The Earth never runs out of usable energy because it constantly dumps its useless energy to space, and receives new usable energy from the sun. And the sun has an enormous supply of usable energy. Current calculations suggest that it could continue to supply the Earth with usable energy for at least 5 billion years into a hypothetical future. That’s a lot of usable energy. But it’s not infinite. Yet humans were originally created to be immortal – to live forever. What would they have done when the sun runs out of usable energy as required by the second law?
Perhaps humans would leave the planet when the sun dies, and colonize other worlds around other stars. But eventually, those stars also must exhaust their usable energy. Since the universe is, by definition, an isolated system, the total usable energy in the universe must eventually go to zero. How then could human beings possibly live forever in a universe that cannot last forever? And so, some creationists have proposed that this wasn’t a problem before sin because the second law was not in operation yet. But we have seen biblical evidence to the contrary. And given that our body chemistry is designed to harness the second law apparently from creation, this doesn’t seem to be a reasonable answer. What then is?
It may not be possible for us to know for sure what the answer is. But two possible solutions present themselves. The first is that, had Adam and Eve not sinned, God would occasionally transform some of the unusable energy back into a usable form. He might do this at certain intervals, or perhaps continuously in certain locations in space. Perhaps there was a balancing principle before sin whereby entropy was reduced in certain locations or at certain times, such that the net entropy of the universe is unchanged over time.
Alternatively, we should remember that in God’s sovereignty and omniscience, there was no need for a contingency in the event that Adam and Eve would not sin. God knew from the beginning that Adam and Eve would sin, and so there was no need to make the original universe in such a way that it could last forever without running out of usable energy. It has plenty of usable energy for God to demonstrate His mercy to repentant sinners, to carry out His plan of redemption. Whatever option God chose, clearly the second law of thermodynamics being in operation from creation is not an unsolvable problem.
Apologetic Implications
We have seen that there are some apologetic arguments that misuse the second law. But not all. The second law does imply that the universe has a beginning, as we will see below. And this has apologetic implications because it disallows any worldview in which the universe is eternal.
The second law of thermodynamics states that the entropy of an isolated system can only increase – that energy will go from a usable state to an unusable state until all the energy is unusable. The universe is necessarily an isolated system because it is defined to be everything physical that exists. Yet we observe that there is a lot of usable energy in the universe. So, if the universe were eternal, then all the energy would have already decayed to an unusable state. In other words, if the universe were infinitely old, it would have exhausted its usable energy long ago. Since that has not occurred, the universe cannot be infinitely old. Therefore, the universe had a beginning.
Since the universe has a beginning, it requires a cause. The law of cause-and-effect demands this. In the Christian worldview, God is the cause of the universe. So the Christian worldview can make sense of the implications of the second law. But the atheist has a problem – an effect with no cause. Some atheists might attempt to alleviate the problem of the cause of the universe by postulating that the universe is eternal. After all, if something has no beginning, then it cannot have a cause. This solution is logically self-consistent, but is not consistent with science. It defies the second law of thermodynamics.
The eternal universe view is no longer popular, but it does exist. Most secularists believe that the universe had a beginning. The most common secular position on origins – the big bang – states as much. But if you encounter someone who believes in an eternal universe, the second law of thermodynamics is a sound way to refute such a concept.
The Cosmological Argument
The second law also has relevance to a popular argument for the existence of God known as the cosmological argument. The argument goes something like this: (1) Anything that has a beginning has a cause. (2) The universe has a beginning. (3) Therefore, the universe has a cause. (4) That cause is God. The first three steps in this argument are perfectly legitimate. The first two premises are true, and the third proposition follows logically from them. Getting from the third proposition to the fourth is the questionable leap and will require extra steps which are not included in this summary. I am not convinced that these extra steps actually establish the truth of the conclusion without tacitly presupposing the truth of Christianity. But I do think that the cosmological argument has confirmatory power within the Christian worldview; it makes sense that God created the universe, although the argument doesn’t necessarily prove it. In any case, we will not examine the legitimacy of these extra steps at this time, but will focus only on the first three steps of this proof.
To convince an unbeliever of the third proposition, we must establish the truth of the first two. The third will follow inescapably from them. The first proposition is a requirement of the law of cause-and-effect. Few people would deny this since we rely upon the law of cause-and-effect everyday for our survival. However, some Christians make a dreadful mistake in their attempt to prove the second proposition. They appeal to the big bang.
Namely, many Christians will argue that the universe has a beginning because the big bang states as much. But the big bang is a false origin story. As we have shown previously, it is contrary to the Genesis account, is not good science, and has abundant scientific problems. We should not accept as truth that which is false in order to persuade someone of the truth.[6] If the big bang were true, then the Bible is false. And so a cosmological argument that is based on the big bang might (at best) prove the existence of a god, but it could never prove the existence of the biblical God since it is contrary to the Bible.
And it is unnecessary. The second premise can be demonstrated by appealing to the second law of thermodynamics, which is true and is observed to be true. So, the second law is a legitimate and logical way to demonstrate that the universe has a beginning, and doesn’t require us to embrace anything contrary to the Bible. The second law of thermodynamics confirms the biblical truth that the universe has a beginning.
[1] Neglecting relativistic effects. The formula is not actually the definition of kinetic energy, but an approximation that is extremely accurate for velocities much less than the speed of light. For kinetic energy at higher velocities, see the book The Physics of Einstein.
[2] For clarity, we are neglecting the infinitesimal amount of energy lost to air resistance.
[3] The first law of thermodynamics is easy to understand and makes sense in light of Scripture. God alone has the power of creation (John 1:3), and since He ended His work of creation by the seventh day (Genesis 2:2), no new energy will come into existence. Furthermore, since God upholds all things by the expression of His power (Hebrews 1:3), energy will not simply cease to exist.
[4] For the sake of simplicity, I am neglecting the small amount of energy that is carried away by sound waves, and that which is transferred to the concrete.
[5] Of course, a few photons from the ice would go to the coffee, but since the coffee is hotter, it releases for more photons. Therefore, the net flow of energy will be from the hotter coffer to the cooler ice cube.
[6] I will grant that a reductio ad absurdum would be a legitimate approach. This is where we temporarily assume the incorrect position of our opponent to show that such a position leads to absurdity. Namely, we could say, “By your own view (the big bang which I reject) the universe has a beginning, which requires a cause. But you have no cause. Your position is irrational.” This method exposes the absurdity of the unbeliever’s position without actually embracing anything that is false.